Newsletter
The latest newsletter includes what’s happening with monitoring, funding, nurseries, education, the calendar of events, and more. Looking for something older? View the newsletter archive. Receive the newsletter directly in your inbox by signing up for our mailing list.
Current California Oak Mortality Task Force Newsletter: August 2024
(Printable COMTF Report August 2024)
Mangement – California
After two wet winters, Phytophthora ramorum spread is apparent in Humboldt Redwoods State Park. Until recently, this state park, one of the largest in the California State Parks system, represented the northernmost extent of continuous P. ramorum infestation along the Highway 101 corridor in Southern Humboldt County, with confirmed infections limited to trees within the core of the park, south and east of Bull Creek and along the South Fork Eel River. However, at the end of this winter, symptoms were observed on tanoaks on the north side of the creek. These symptoms bordered a complex of prairies in the area where for many years an active program of prescribed burning has kept the prairies open by killing encroaching Douglas-firs (Fig. 1). Subsequent to initial P. ramorum sampling near the bottom of the system of prairies, a ground survey by staff from State Parks, UC Cooperative Extension, and CAL FIRE was undertaken in late May to ascertain how far P. ramorum had spread uphill and whether symptomatic forest areas might be added to the long-term program of repeated prescribed burning.


The survey found P. ramorum symptoms scattered up to the top of the ridge (Peavine Ridge), and almost all the sampled symptomatic material yielded P. ramorum, as confirmed by the California Department of Food and Agriculture (CDFA), Plant Pest Diagnostics Center. Troublesomely, one infected tanoak sprout found near the top of the ridge was located in an open forest of very large conifers and tanoaks that includes one of the largest known tanoak trees. The symptomatic sprout is not near a roadside or a stream. Positive California bay laurels were generally located near streams scattered along the slope. Look-alike symptoms on bay laurel, tanoak, and madrone were confirmed by CDFA as being caused by Calonectria californiensis, Apiognomonia errabunda (= Discula quercina), and Diaporthe sp., respectively. To help facilitate cultural and ecological goals, discussions to include P. ramorum-infested areas in the long-term prescribed burning goals for this part of the park will continue, as will continued sampling and monitoring. The discovery of P. ramorum near large, culturally important tanoak trees is a reminder of one of the most concerning aspects of the continued spread of this pathogen and of long-term ecological impacts associated with this disease. The pathogen’s spread with a complex of look-alike pathogens that also likely spread during heavy late-winter precipitation underscores the continuing challenge of monitoring P. ramorum under a changing climate regime with the potential to swing ever more erratically between wet and dry extremes. For more information contact Wallis Robinson, wlrobinson@ucanr.edu.
Oregon – Management, Monitoring, and Regulation
To date in 2024, 14 new P. ramorum infestations have been detected at or beyond the Oregon Generally Infested Area (GIA). Using a 300-600 ft treatment buffer, 2024 treatment areas total approximately 388 acres of private land, 42 acres on State Park lands, and 51 acres on U.S. Forest Service lands. Oregon’s P. ramorum stream baiting program commenced in late April with 64 stream drainages in and outside of the quarantine area to be monitored this year. Six stream drainages have tested positive for P. ramorum so far this year. Ground surveys are being planned for the new positive drainages along the north bank Rogue River and a stream that drains into the Elk River east of Port Orford.
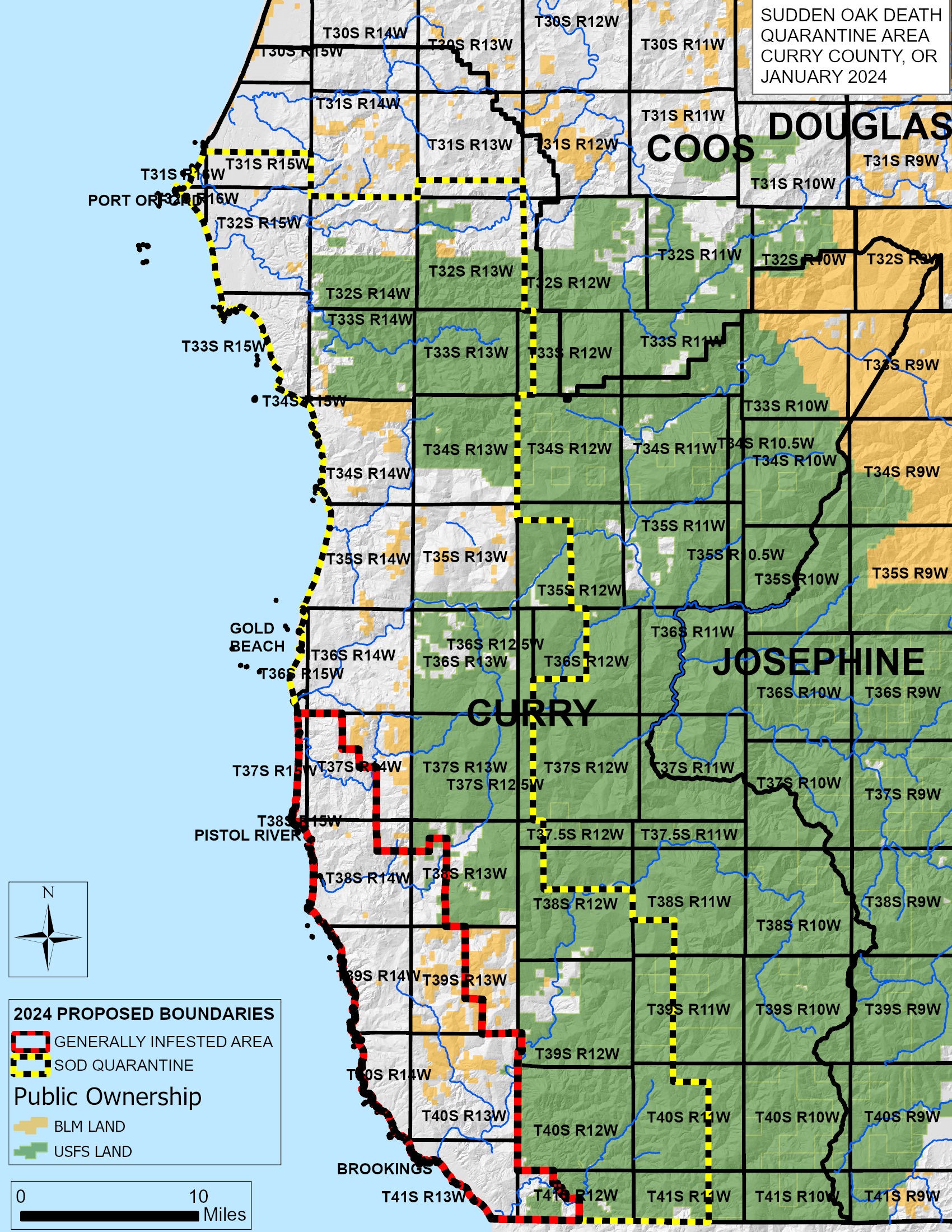
Progress for Oregon’s Slow-the-Spread Program for the management of sudden oak death is updated on a monthly basis on the Oregon SOD Program Dashboard. Since the start of the sudden oak death program, the Oregon Department of Agriculture (ODA) has overseen the expansion of the quarantine boundary to account for the spread of P. ramorum/sudden oak death on the landscape. Despite several new infestations being outside the current official quarantine area, those areas are covered under the current ODA SOD (sudden oak death) rules, which establish a quarantine on any area in the state where a SOD infestation occurs (OAR 603-052-1230(2)(d). But given the 23 new detections outside of the 2015 SOD quarantine boundary since 2021, SOD program staff propose to expand the quarantine and GIA. The new proposed quarantine area is 901 square miles or 45% of Curry County, an increase in area of 15%. The proposed GIA area is 178 square miles (see map, Fig. 3). For more information contact Sarah Navarro, Sarah.Navarro@usda.gov.
Nurseries and Managed Landscapes
Five P. ramorum positive nurseries have been confirmed in California to date in 2024. Six nurseries in California that were previously positive for P. ramorum underwent enhanced inspections in March and April. Of the six previously positive nurseries, three nurseries were found positive for P. ramorum again in 2024 and are undergoing the USDA Protocol for Interstate Nurseries Confirmed Positive for Phytophthora ramorum. The other two 2024 positive nurseries were found during trace forward inspections or annual inspections. A trace forward inspection yielded additional positive plants at one retail nursery in a quarantine county. The fifth nursery, not previously positive, was found positive during an annual inspection. These two nurseries are undergoing the P. ramorum retail protocol for positive nurseries.
Plants confirmed at California nurseries found positive for P. ramorum in 2024 are: Arbutus x ‘Marina’ (1); Camellia sasanqua ‘Shishi Gashira’ (1); Cornus capitata ‘Mountain Moon’ (1); Cornus capitata ‘Porlock’ (1); Cornus capitata (1); Cornus kousa var. chinensis (1); Hamamelis virginiana (1); Loropetalum chinense (134); Loropetalum chinense var. rubrum (3); Rhododendron sp. (3); Rhododendron sp. ‘Aurora’ (2); Rhododendron sp. ‘Firestorm’ (2); Rhododendron sp. ‘Holden’s Solar Flair’ (3); Rhododendron sp. ‘June Pink’ (1); Rhododendron macrophyllum ‘Pacific Rhododendron’ (1); and Viburnum ‘Mariesii’ (2).
Arbutus x ‘Marina’ is not on the USDA APHIS P. ramorum host or associated host list. Koch’s postulates need to be completed prior to it being added to the host list. For more information contact Carolyn Lambert, Carolyn.Lambert@cdfa.ca.gov.
Washington State Department of Agriculture concluded trace-forward investigations from a March detection. The out-of-state nursery shipment affected 70 homeowners. In total, 13 tissue samples and two soil samples were collected. All samples tested negative for P. ramorum. For more information contact Haley Palec, hpalec@agr.wa.gov.
Taxonomic Challenge to the Genus Phytophthora
Phytophthora is an ancient, historic, biologically and structurally cohesive and evolutionarily successful genus, serving as the founding pathogen for the discipline of plant pathology. The genus comprises more than 250 species across at least 16 clades. The downy mildews are also phylogenetically nested within Phytophthora, rendering it cladistically paraphyletic. A paraphyletic group is one where more than one group descended from a common ancestor.
For more than two decades, despite being aware of this paraphyly, the Phytophthora taxonomic community has declined to make any alterations to the status of the genus, in some cases actively embracing the status quo and advocating for the maintenance of the paraphyletic genus as-is. Despite this, a member of the downy mildew systematic community recently began the process of breaking up Phytophthora into as many as 18 genera. A virtual meeting was convened in April, under the auspices of the American Phytopathological Society, to assess the scientific support for retaining the name Phytophthora for all major clades of the genus. The workshop explored the evolutionary, biological, taxonomic, regulatory, social and economic ramifications involved with altering the current definition of Phytophthora. Following the sessions, break-out discussions were held among attendees, and attendees were able to cast votes regarding the nomenclatural status of the genus. Following an overwhelming consensus by attendees, a letter to the editor of the journal Phytopathology is being drafted as part of the formal process to retain the current definition of this critically important genus. For more information contact Tyler Bourret, Tyler.Bourret@usda.gov.
Research
Bye, R. 2023. Exploring the epigenetic response of Larix kaempferi to Phytophthora ramorum infection. PhD thesis. Aberystwyth University, Wales. 354 pgs. https://research.aber.ac.uk/files/75528624/Bye_Ruby.pdf.
Condensed abstract: This study explored changes in cytosine methylation in Larix kaempferi (larch) seedlings infected with the invasive oomycete pathogen Phytophthora ramorum. This forest pathogen has caused the death of millions of trees worldwide, and significantly disrupted the UK forestry industry. Several loci showing methylation variability upon infection were identified. However, further work is needed to optimize the bioinformatic analysis before epimarks identified through this pipeline can be reliably used for diagnosis.
Dort, E. 2024. Forest pathology in the genomics era: Combining comparative genomics and CRISP-CAS9 gene editing to gain new insights into the genetics of filamentous plant pathogens. University of British Columbia. PhD thesis. https://open.library.ubc.ca/media/download/pdf/24/1.0441005/4.
Condensed abstract: A part of this work focused on developing molecular techniques for filamentous forest pathogens in the oomycete genus Phytophthora. First, a polyethylene glycol-mediated plasmid transformation method was tested in five forest Phytophthoras to determine which species was most amenable to further molecular method development. Next, CRISPR-Cas9 gene editing was tested in two forest Phytophthoras, P. cactorum and P. ramorum, using a plasmid-ribonucleoprotein co-transformation approach. The results from the plasmid transformations and CRISPR-Cas9 gene editing demonstrated both the potential and challenges for developing molecular techniques in Phytophthora species and provide a foundation for future genomic research in forest Phytophthoras.
Foster, Z.S.L.; Tupper, A.S.; Press, C.M.; Grünwald, N.J. 2024. Krisp: A Python package to aid in the design of CRISPR and amplification-based diagnostic assays from whole genome sequencing data. PLoS Computational Biology. 20(5): e1012139. https://doi.org/10.1371/journal.pcbi.1012139.
Abstract: Recent pandemics like COVID-19 highlighted the importance of rapidly developing diagnostics to detect evolving pathogens. CRISPR-Cas technology has recently been used to develop diagnostic assays for sequence-specific recognition of DNA or RNA. These assays have similar sensitivity to the gold standard qPCR but can be deployed as easy to use and inexpensive test strips. However, the discovery of diagnostic regions of a genome flanked by conserved regions where primers can be designed requires extensive bioinformatic analyses of genome sequences. We developed the Python package krisp to aid in the discovery of primers and diagnostic sequences that differentiate groups of samples from each other, using either unaligned genome sequences or a variant call format (VCF) file as input. Krisp has been optimized to handle large datasets by using efficient algorithms that run in near linear time, use minimal RAM, and leverage parallel processing when available. The validity of krisp results has been demonstrated in the laboratory with the successful design of a CRISPR diagnostic assay to distinguish the sudden oak death pathogen Phytophthora ramorum from closely related Phytophthora species. Krisp is released open source under a permissive license with all the documentation needed to quickly design CRISPR-Cas diagnostic assays.
Leal, I.; Feau, N.; Uzunovic, A.; Foord, B; Hamelin, R. C. 2024. A molecular method to assess viability of Phytophthora in infected wood following heat treatment. PhytoFrontiers. https://doi.org/10.1094/PHYTOFR-05-24-0056-R (First Look.)
Abstract. International trade in wood products is an important component of the global economy. However, wood and wood products may have pests associated with them that could be introduced into importing countries, posing phytosanitary risks, and leading to the implementation of regulatory restrictions that affect wood trade. The application of heat to kill wood-associated pests has been a successful phytosanitary method to reduce their spread. To evaluate the efficacy of wood heat treatment to kill fungal and fungus-like pathogens, the method of choice has been to grow organisms in cultures for subsequent identification. However, some plant pathogens can be difficult or impossible to grow in axenic cultures and a molecular method can still be useful for assessing pathogen viability after heat-treatment. RNA is a single stranded molecule that is responsible for the transcription of genes. Since it becomes rapidly unstable after cell death, it provides a measure of viability. We therefore designed and tested RNA-based molecular diagnostic assays targeting essential genes and assessed their presence after heat treatment in wood colonized by four Phytophthora species of phytosanitary concern (P. xmultiformis, P. cinnamomi, P. lateralis and P. ramorum) through reverse transcription and real-time polymerase chain reaction (RT-qPCR). Our assays differentiate between genomic and mRNA as the TaqMan probes span exon-intron junctions. We validated these RT-qPCR assays to assess heat treatment efficacy of Phytophthora-inoculated wood. These assays can be very useful tools to assess effectiveness of current and emerging phytosanitary wood treatments.
López-Garcia, N.; Romeralo, C; Rönnberg, J. and Witzell, J. 2024. Control and management of Phytophthora damage in forestry—a systematic mapping study. Forest Pathology. 54(4): e12878. https://doi.org/10.1111/efp.12878.
Abstract: Plant pathogens in the genus Phytophthora are a severe threat to forest plantations, ecosystems and tree nurseries. Especially in forests and natural ecosystems, there is a lack of effective measures to control and manage these pathogens. In this study, we conducted a systematic mapping review to collate evidence regarding the control and management of forest Phytophthora in different production settings and ecosystems. The study aimed to reveal possible knowledge gaps, thus guiding future research priorities. We extracted information from nine databases, limiting the search to studies published during the time period from January 2010 to December 2022. The articles were shared between three reviewers who classified the reports using a set of inclusion/exclusion criteria. A total of 561 articles were included and mapped in a database using pre-defined coding, and critically appraised for relevance and reliability. The analysis showed that biological or bio-based measures were the most studied interventions, followed by genetics or breeding programmes, whereas chemical and silvicultural management approaches were less studied. Most of the studies were conducted in Europe, North America, Australia and New Zealand. Phytophthora cinnamomi has been the most studied species followed by P. ramorum. We discuss the current knowledge gaps in the implementation of existing research, likely due to a lack of holistic understanding of the processes over time and space, and suggest future research that is needed to manage Phytophthora in forest ecosystems.
Related Research
Bačová, A.; Cooke, D.E.; Milenković, I.; Májek, T.; Nagy, Z.Á.; Corcobado, T.; Randall, E.; Keillor, B.; Cock, P.J.; Jung, M.H. and Jung, T. 2024. Hidden Phytophthora diversity unveiled in tree nurseries of the Czech Republic with traditional and metabarcoding techniques. European Journal of Plant Pathology. doi.org/10.1007/s10658-024-02886-1.
Laurence, M.H.; Mertin, A.A.; Scarlett, K. [and others]. 2024. Phytophthora in urban tree planting stock: Are we managing the risk to the urban forest and natural ecosystems? Plant Pathology. https://doi.org/10.1111/ppa.13960 (Early View).
Paap, T.; Balocchi, F.; Burgess, T.I.; Bose, T.; Wingfield, M.J. 2024. A diverse range of Phytophthora species from botanical gardens in South Africa, including the novel Clade 5 species, Phytophthora mammiformis sp. nov. Fungal Systematics and Evolution. 13: 111–122.
Education
Ecology & Impacts of Emergent Forest Diseases in California. Matteo Garbelotto’s UC Berkeley class from spring 2024 is now available free and online. Recommended reading, lecture recordings, slides, even essay topic suggestions are posted. Subjects covered include several high impact forest diseases, molecular diagnostics, disease control and prevention
Meetings – Save the Date
The fall meeting of the California Oak Mortality Task Force will be held virtually on Tuesday, October 29, 2024, from 1 pm to 3 pm PDT. The meeting will focus on the status of sudden oak death/P. ramorum in California and Oregon wildlands.
The Phytophthoras in Native Habitats Work Group will meet virtually on Wednesday, October 30, 2024, from 1 pm to 3 pm PDT. The meeting theme is “Threats to California Native Plants.” Talks will explain risks to plant health from viruses, excessive heat, and other concerns.
Both meetings are free, and open to all interested parties, but registration is required. The meetings will be recorded and posted to www.suddenoakdeath.org. Complete agendas and more information will be available soon at www.suddenoakdeath.org; for questions contact Janice Alexander, jalexander@ucanr.edu.
Farewell
Best wishes upon the retirement of Pat Shiel, USDA APHIS, Plant Protection and Quarantine, P. ramorum regulatory program. Pat worked for APHIS on various aspects of P. ramorum for over 20 years including deployment to California in 2004 during the P. ramorum federal emergency program. Pat concluded his USDA career as the Science and Technology representative of the APHIS Plant Protection and Quarantine, P. ramorum regulatory program cross functional working group. In that capacity, he provided broad scientific support to fulfill the requirements of the federal regulations and advance P. ramorum scientific knowledge.


